Quality Assurance-Physical
Friability Testing
Friability is the tendency for a tablet to chip, crumble or break following compression and can occur due to shock or abrasion during subsequent manufacturing steps, packaging or shipping. Normally limited to uncoated tablets and surfaces, it is a tendency that can significantly erode tablet quality. Tablets need to be hard enough so that they do not break up in the bottle, to safeguard product stability and dose uniformity, but sufficiently friable to disintegrate in the gastrointestinal tract and release the active(s).
Chapters Ph. Eur. 2.9.7 and USP <1216> describe reproducible and standardised methods and equipment to determine the friability of a tablet.
A standard friability drum has an inside diameter of 287 mm and a depth of 38 mm. Each drum is fitted with a curved baffle which subject tablets to a drop of 156 mm during rotation. The sample (normally 10 tablets) to be tested is first weighed and then placed into the drum. The drum is then rotated 100 times at a speed of 25 rpm. Any loose dust from the sample is removed and the sample re-weighed. Friability of a sample is defined in terms of percentage (%) weight loss. A maximum weight loss of no more than 1% is considered acceptable for most tablets.
Attrition caused by tablets rubbing together can also be measured using this method with a special abrasion drum. For coated tablets, granules and spheroids, friability cannot be determined using a conventional friability tester, as the dosage form is too hard for meaningful weight loss measurements. Alternative equipment that oscillates the sample at high frequencies is available for this purpose (see Ph. Eur. 2.9.41). The abrasive forces generated by the horizontal shaking movement of the oscillating arm of this equipment allows users to easily optimise test conditions for each type of formulation with simple-to-use programmable controls.

Advanced friability testers for all tablet types
Providing highly reproducible and cost-efficient friability testing, the FRVi series of friability testers from Copley allow analysts to easily characterise the critical tendency of tablets to chip, crumble or break.
Based on an original design by Roche, the Copley FRVi Series friability tester is an established standard within the pharmaceutical industry. Equipped with advanced design features, ranging from convenient sample loading and unloading to an integrated friability calculator, the FRVi Series streamlines the workflow associated with routine friability testing. Copley FRVi Series features 1 drum (FRV 100i) and 2 drums (FRV 200i) test station capacity.
FRVi Series: Key Features
- Intuitive touchscreen control with icon-based menu structure simplifies operation and clearly displays test parameters throughout run.
- Integrated friability calculator removes the need for manual tablet weight loss calculations, reducing the risk of analyst calculation error and improving data accuracy.
- Robust metal case with advanced corrosion protective coating.
- Compatible with friability and/or abrasion drum(s).
- Small unit footprint saves previous benchtop space.
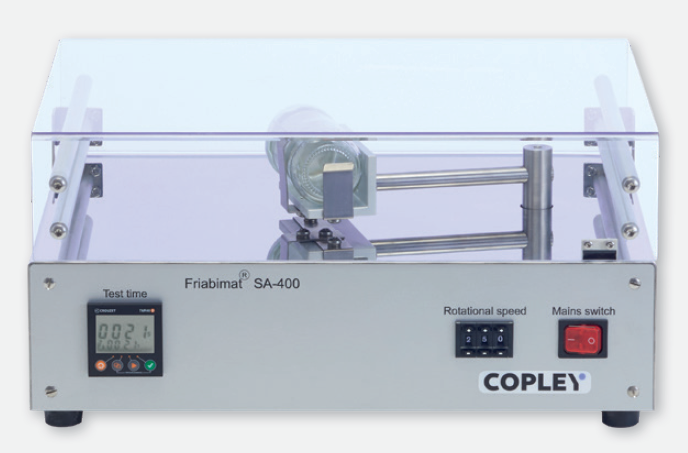
For the hardest and most robust solid dosage forms which fall outside the scope of the conventional friability tester a higher-energy input instrument is needed to generate quantifiable changes in surface mass. The Friabimat, as described in Ph. Eur. 2.9.41, has been designed to generate the necessary abrasive forces required to define the friability characteristics of these types of dosage forms.
Not only useful for determining the friability of hard pellets and granules, the Friabimat is suitable for detecting variations in mechanical properties between different formulations and batches, making it a convenient tool for both research and development and quality control applications.
Friabimat SA-400 : Key Features
- Quantifiable abrasion of hard tablets, granules and pellets
- Clear acrylic lid with magnetic interlock to ensure safe operation
- Stainless steel case ideal for production environments
- Option: Oscillation frequency verification certificate

