Lateral Flow
RapidChek System
The test system utilizes enrichment media coupled to an advanced lateral flow immunochemical technology to detect pathogen in various foodstuff and environmental applications providing fast, accurate and reliable results.
Benefits:
- Fast & simple procedure
- Reliable results
- Easy resource management
- Approved by AOAC RI
Fast & Simple Procedure
- Next day results
- Simplified media preparation
- Minimal training
- No additional equipment
Easy Resource Management
- High scalability
- Kit storage at room temperature
- Long shelf life
Reliable Results
- AOAC approved
- AFNOR validated
- NPIP approved
Applications
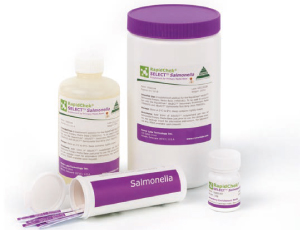
RapidChek® SELECT™ Salmonella has been designed to detect the pathogen in meat and dairy products, seafood and vegetable products, eggs, feedstuffs, as well as environmental samples.
How the Test Works
The method uses a novel application of bacteriophages (or phage) in the media to act as selective agents, enhancing both the specificity and sensitivity of the overall method. This patented media system is used in combination with a next generation RapidChek® SELECT™ lateral flow detection device.
It contains a proprietary panel of anti-Salmonella antibodies engineered to enhance the overall performance of the method.
Validations
The method is AOAC and AFNOR certified, and NPIP approved (specifically for use in poultry house environmental samples).
- Faster product release
- Fewer recalls
- Lower total cost in use
Increasing lab productivity :
- Reduced material and labor costs
- Simple application minimizes
- training costs
- Less errors through easier handling
Applications
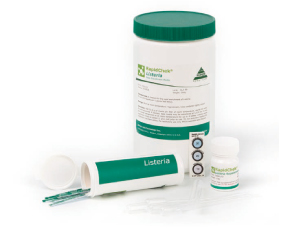
The RapidChek® Listeria method is designed to detect Listeria spp on environmental surfaces, a variety
of ready-to-eat foods, dairy products, and fish products. The test kit permits the presumptive
detection and identification of the target pathogen by a one step process in 40 hours.
How RapidChek® Listeria Works
The test is used in combination with a single enrichment broth for a rapid test with no transfers. The
RapidChek® Listeria media system consists of two components, a base media and a supplement. After
enrichment, the sample is dispensed into a test tube and heated for 5 minutes. The test strip is added
directly to the sample tube. Results are obtained within 10 minutes. One line indicates a negative
result while two lines indicate a positive result.
Validations
RapidChek® Listeria is AOAC approved for use in a variety of foods and environmental samples.
Fast & simple procedure
- Next day results
- Simplified media preparation
- Minimal training • No additional equipment
Easy resource management
- High scalability
- Kit storage at room temperature
- Long shelf life
Reliable results
- AOAC approved
Applications
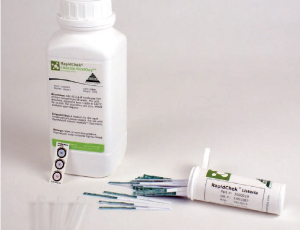
The RapidChek® Listeria NextDay™ method is designed to detect Listeria spp. on environmental surfaces and a variety of food matrices. The test kit permits the presumptive detection and identification of the target pathogen by a one step process in 24 – 48 hours.
How the Test Works
The RapidChek® Listeria NextDay™ technology couples a sensitive immune-detection strip with a single proprietary enrichment media for fast and accurate Listeria species results within 24 – 48 hours. The immune-detection strip employs a unique combination of proprietary Listeria antibodies and a colloidal gold conjugate coated on the surface of a membrane. After enrichment, the sample is transferred into a provided test tube and heated for 5 minutes. The immuno-detection strip is then added directly into the sample and within 10 minutes results are read. One line indicates a negative result and two lines indicate a positive result.
Validations
RapidChek® Listeria NextDay™ is AOAC approved for use on various environmental samples (stainless steel, painted concrete, rubber, and plastic) and food samples (hot dogs, roast beef, frozen breaded chicken, frozen meatballs, whole milk, ice cream, ricotta cheese, shredded mexican cheese and cheese powder).
- Faster product release
- Fewer recalls
- Lower total cost in use
- E. coli O157:H7 verification from RapidChek® media enrichment by PCR
Increasing lab productivity :
- Reduced material and labor costs
- Simple application minimizes training
- costs
- Less errors through easier handling
- Validated with 25 g to 375 g beef
- samples
Applications
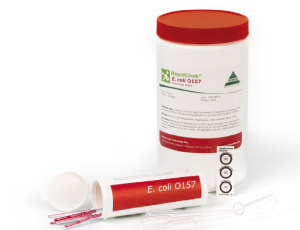
RapidChek® E. coli O157 (Including H7) has been designed to detect the pathogen in meat, dairy, and plant products.
How RapidChek® E. coli Works
The RapidChek® immunoassay employs a unique combination of anti-pathogen antibodies and colloidal gold conjugate coated on the surface of a membrane. The RapidChek® assay can be used in
combination with a proprietary RapidChek® enrichment media broth for a rapid 8-hour result for a 25 g sample or 10-hour and 12-hour rapid result for 375 g samples. A second protocol using a standard 15-hour enrichment in modified TSB broth plus novobiocin is also available. After suitable enrichment,
the sample broth is transferred to a test tube and a test strip is inserted into the tube. No heat treatment is required. Within 10 minutes you have a result. One line means the sample is negative, two lines mean it’s positive. It is the fastest definite negative result.
Validations
The method is AOAC approved for use in ground beef and boneless beef trim.
- Isolate E. coli O26, O45, O103, O111, O121, O145 and O157 present within an enriched sample
- Increases the chances of identifying a “Big Six” non-O157 and O157 STEC from selective agar plates
- Easy to use technology requiring no additional instrumentation
- Validated: Listed in the USDA FSIS MLG 5B.04 for the confirmation of non-O157 STECs
How the Test Works
The RapidChek® CONFIRM™ STEC IMS confirmation kit uses specific, high-affinity antibodies each attached to separate magnetic particles. Each coated magnetic particle solution is added to a potential positive enrichment and incubated. If any of the STECs are present, it will bind to the magnetic particles via the antibody. A magnet is then used to concentrate the bound magnetic particles and the remaining enrichment is discarded. When the sample is plated to selective agar for confirmation, E. coli O26, O45, O103, O111, O121, O145 and O157 are the predominant colonies that form increasing the likelihood of picking that organism from a plate. By reducing the presence of other bacterial species, the accuracy and repeatability of the cultural confirmation is increased and the labour and time decreased.
Validated Applications
The RapidChek® CONFIRM™ STEC IMS Kit is approved by the USDA FSIS in MLG 5B.04 for use in the confirmation of E. coli O26, O45, O103, O111, O121, O145 or O157 from potential positive beef samples.

Fast & Simple Procedure
- Fast product release
- Simplified media preparation
- Minimal training
- No additional equipment
Easy Resource Management
- High scalability
- Kit storage at room temperature
- Long shelf life
Reliable Results
- AOAC approved
- FDA equivalency
Applications
RapidChek® SELECT™ Salmonella Enteritidis has been designed to detect the pathogen in drag swabs, egg pools, and chicken carcass rinses.
How the Test Works
The method uses a novel application of bacteriophages (or phage) in the media to act as selective agents, enhancing both the specificity and sensitivity of the overall method.
This patented media system is used in combination with a next generation RapidChek® SELECT™ Salmonella Enteritidis test strip. It contains proprietary anti-Salmonella D1 serogroup antibodies engineered to enhance the overall performance of the method. After 10 minutes, if Salmonella Enteritidis is present in the sample, two red lines will form. One line indicates a negative result.
Validations
The method is AOAC approved and awarded FDA equivalency.

The RapidChek® CONFIRM™ IMS Kit is designed to be used with the RapidChek® SELECT™ Salmonella Enteritidis Test System when confirming the presence of Salmonella Enteritidis in high burden samples such as poultry house environmental drag swabs.
- Concentrates SE present within an enriched sample
- Increases the chances of identifying SE from selective agar plates
- Easy to use technology requiring no additional instrumentation
- Validated: AOAC-RI and US FDA for environmental drag swabs
How the Test Works
Within an enriched environmental drag swab sample, there are mixed populations of Salmonella species typically present, creating a challenging environment for isolation of Group D1, including SE isolates. The use of the RapidChek® CONFIRM™ IMS kit reduces the presence of other non-SE, Salmonella species through washing steps and concentrates the Group D1 species. When the sample is struck to selective agar plates for confirmation, Group D1 Salmonella are the predominant colonies that form increasing the chances of picking SE if the organism is present within the sample. By reducing the presence of other non-SE Salmonella species, the accuracy and repeatability of the cultural confirmation is increased and the labour and time is decreased.
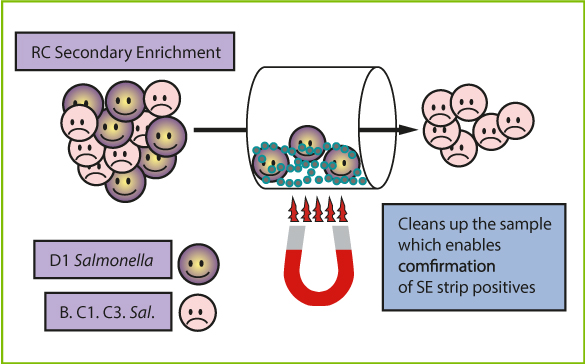
Validated Applications
The RapidChek® CONFIRM™ Salmonella Enteritidis IMS Kit is AOAC approved for use of the confirmation of SE presumptive positive poultry house environmental drag swabs
- Days faster than ISO, USDA or FDA Method
- Convenient one step enrichment
- Minimal training
- No additional equipment
- Unlimited scalability
- Storage at room temperature
- Minimal training
- Long shelf life
- AOAC approved
Applications
The RapidChek® Listeria monocytogenes method is designed to detect Listeria monocytogenes on environmental surfaces and a variety of food matrices. The test kit permits the presumptive detection and identification of the target pathogen by a one step enrichment in 44 – 48 hours.
How the Test Works
The RapidChek® Listeria monocytogenes system couples a sensitive immune-detection strip with a single proprietary enrichment media for fast and accurate Listeria monocytogenes results within 44 – 48 hours. The immune-detection strip employs a unique combination of proprietary Listeria monocytogenes antibodies and a colloidal gold conjugate coated on the surface of a membrane. After enrichment, the sample is transferred into a provided test tube and the immuno-detection strip is added directly into the sample. Within 10 minutes results are read. One line indicates a negative result and two lines indicate a positive result. The test kits are stored at room temperature.
Validations
RapidChek® Listeria monocytogenes is AOAC approved for use on various environmental surfaces (stainless steel and plastic) and food samples (hot dogs, frozen breaded chicken, frozen cooked shrimp, cured ham, and ice cream).
- Aerobic enrichment
- Simplified media preparation – no additional supplements
- Minimal training
- No specialized equipment
- Unlimited scalability
- Storage at room temperature
- Long shelf life
- AOAC approval pending
Applications
The RapidChek® Campylobacter test system is designed to detect C. jejuni, C. coli and C. lari in carcass rinses. The test kit permits the presumptive detection of the target pathogen in 47 – 49 hours.
How the Test Works
The RapidChek® technology couples a sensitive immune-detection strip for Campylobacter species with a proprietary aerobic enrichment media for fast and accurate results for carcass rinses. The lateral flow device is added directly into a provided tube with an aliquot of the enriched sample. Within 20 minutes results are read. One line indicates a negative result and two lines indicate a presumptive positive result.
Confirmation
Presumptive positive results should be confirmed by a cultural reference method (USDA MLG 41.04). RapidChek® Campylobacter media samples used in the test procedure can be used for confirmation.
- Salmonella spp. - RapidChek SELECT Salmonella
- Listeria spp. - RapidChek Listeria
- Listeria spp. - RapidChek Listeria NextDay
- E. coli 0157H7 - RapidChek® E. coli O157 (including H7)
- E.coli 0157H7 - RapidChek CONFIRM STEC Immunomagnetic Separation (IMS) Kit
- Salmonella enteritidis - RapidChek SELECT Salmonella Enteritidis
- Salmonella enteritidis - RapidChek CONFIRM Salmonella Enteritidis
- Listeria monocytogenes - RapidChek Listeria monocytogenes
- Campylobacter Kit - RapidChek Campylobacter

